BioInteractions launches expanded medical product development partnership offering at MD&M West
Organisations planning to bring a medical device to market should consider the benefits teams gain by using a single high-end consultancy as they navigate the entire product development process
MD&MWest Booth Number 1829
February 3rd, 2023: BioInteractions, a leading UK biomaterial technology company, is launching its expanded Product Pathway Partnership service incorporating bespoke coatings, optimised application and tooling, testing, regulatory support and commercial production, at the world’s largest medical design and manufacturing event, MD&M West. The company, which is responsible for creating and developing the ground-breaking coating technology for medical devices TridAnt®, as well as the widely proven AstutePlus® and Assist™ solutions, is announcing this new service aimed at advancing organisations ability to navigate the complicated medical device market quicker and more efficiently.
Announcing the expansion of the company’s high-end consulting services, Arjun Luthra, Commercial Director of BioInteractions, commented: “Universally, medical product developers and manufacturers navigate a highly regulated and particularly constrained environment. Our Product Pathway Partnership team closely work with you to navigate all areas including fixture design and tooling, biocompatibility testing, optimising the coating application process, regulatory compliance and optimising your commercial manufacturing. We provide guidance along a strategic pathway with a focus on offering a flexible approach to meeting all of the ever-revolving regulatory demands, the aim of which is to get you to market in the most effective way possible.”
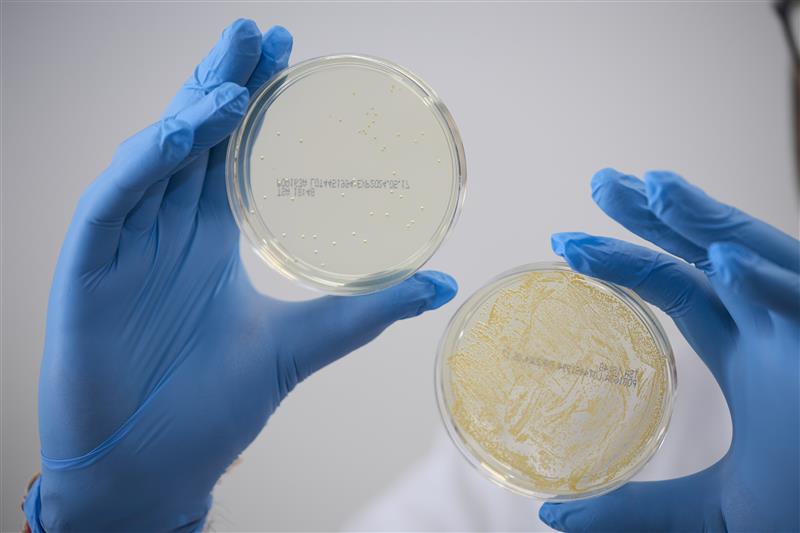
A critical part of ensuring that medical devices meet regulatory requirements is to test for coating efficacy and biocompatibility. During this process, the coating functions are examined to ensure that they are effective, as well as ensuring that the device is durable and safe for patients. As a leading provider of medical coating systems for a wide range of devices, BioInteractions can improve coating efficacy for devices with complex geometries by optimising their materials for use with their broad portfolio of products. Alongside the expanded Product Pathway Partnership, the company’s integrated service streamlines the research and development process of new innovative coated devices using its 30 years of expertise and a range of novel analytical resources to determine the best coating for your device. Furthermore, the company’s 30 years of experience and broad range of products allows it to create bespoke coatings for a wide range of applications. This unique aspect of the service draws in Biointeractions’ expertise and knowledge in applying innovative coatings to a wide variety of devices and enables it to provide innovative solutions for its partners.
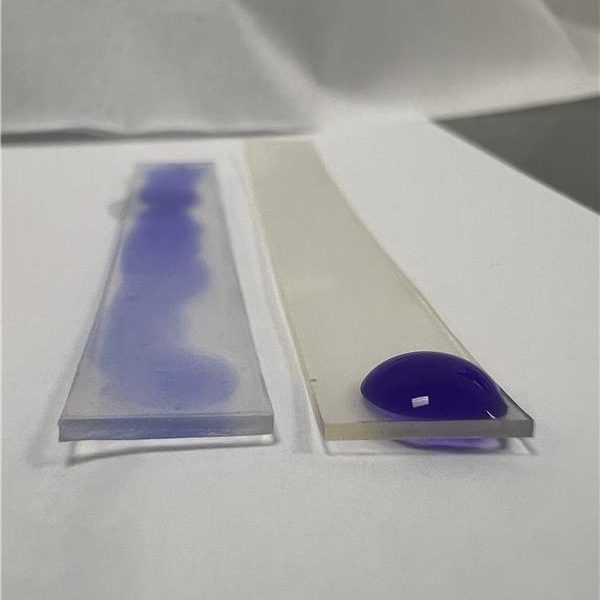
BioInteractions offers various high-performance biocompatible coatings with proven engineered clinical efficacy that expand functionality and broaden design options for engineers including; TridAnt® a non-leaching, safe and durable antimicrobial coating that has been proven effective against a broad spectrum of pathogens including E.coli, MRSA, Flu, Vaccina, Norovirus and SARS-COV. The application of TridAnt® to medical implants reduces the risk of infection, shortens the treatment time and helps to improve the well-being of patients who undergo a range of clinical procedures. The technology is a culmination of three decades of dedicated research and trials, the result of which is a highly-effective material which complies with the latest medical device regulations and has been independently tested by medical device manufacturers to international standards (ISO, EN, PAS). BioInteractions’ innovations continue with AstutePlus® Antithrombogenic Coating, which has been used successfully on chronic implants and on blood-contacting medical devices for over 25 years. In addition to Assist™ Hydrophilic Coating, which is a lubricious and flexible coating that reduces friction, particulate formation and delamination in high-stress and high-movement applications.. They have a long track record of use on devices approved both in the USA and Europe for over 20 years. Overall, coatings on the device enhance biocompatibility of the surfaces, which in turn enables the device to improve the performance of therapy and patient well-being.
The multi-disciplinary team work with a variety of innovators including skilled technicians and scientists, regulatory experts and industry experts. “In an increasingly complex market, rigid thinking is no longer viable for the medical product development process. As advances in medical device manufacturing progress towards more efficient design and quicker manufacturing process, OEMs are highly motivated to find reliable innovations and flexible partners,” explains Jim Veitch, Medical Device Consultant at Synthetic Solutions Group Ltd.
“A cutting-edge team with the insight and proven quality systems in place to help you develop your device is no longer a ‘nice-to-have’ but instead allows you to focus on product development with the confidence that your project requirements will be well managed. BioInteractions’ customers can rely on their state-of-the-art technology as well as their highly knowledgeable team to provide innovative solutions and ease them through the entire product pathway process, enabling a much smoother and effective route to market with unique product offerings,” concludes Veitch.